Antibodies are an essential component of the immune system, allowing the body to protect itself from pathogenic entities through cellular and humoral reactions to different antigens.
While most antibodies produced are naturally polyclonal, resulting in slight variations that alter their antigen specificity, it is possible to produce larger quantities of a single type of antibody. These antibodies are called monoclonal antibodies which all have the same antigen specificity.
-
Save
Over the years, monoclonal antibody development has resulted in many potential drug treatments for immunologic diseases, reversal of drug effects, and cancer therapies. However, despite the potential benefits these monoclonal antibodies can provide, there are still risks of negative side effects. These include acute reactions to their infusions, such as acute anaphylactic and anaphylactoid reactions, serum sickness, tumor lysis syndrome, and cytokine release syndrome.
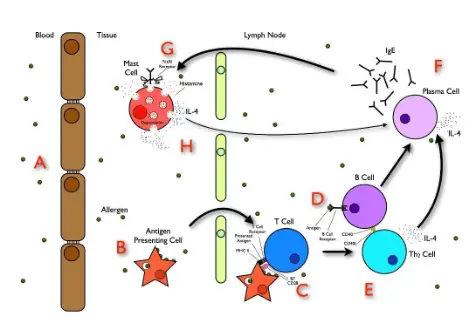
– An acute anaphylactic reaction is a type I immune allergic reaction that involves IgE antibody production against an allergen on first exposure. These antibodies are then attached to the membrane surface of tissue mast cells and circulating basophils. On re-exposure to the allergen, there is a release of preformed mediators including histamine, proteases, proteoglycans, and platelet-activating factors. These mediators cause anaphylactic reactions.
– Anaphylactoid reaction is clinically similar to an anaphylactic reaction, except it occurs through a direct non-immune mediated release of mediators from mast cells and basophils, or through the activation of the direct complement cascade.
– Serum sickness is a type III hypersensitivity reaction that occurs due to the injection of a foreign protein or serum. It involves the formation of immune complexes between the human proteins and the foreign proteins, causing symptoms such as fever, rash, and joint pain.
– Tumor lysis syndrome occurs during the treatment of cancer, where massive tumor cells are destroyed. This causes the release of large amounts of potassium, phosphate, and nucleic acids into circulation. This can lead to many complications including hyperuricemia and hyperphosphatemia causing acute renal injury.
– Cytokine release syndrome is an acute systemic inflammatory syndrome that activates T cells and other immune effector cells in response to immune therapy. This syndrome causes an increase in circulating inflammatory cytokines, which further increases the activation of T cells, macrophages, and endothelial cells. Taken together, cytokine release syndrome can cause severe fever and multiple organ dysfunction.
-
Save
With these potential complications in mind, steps must be taken to minimize immunogenicity. New monoclonal antibody treatments go through vigorous preclinical testing to ensure the safety of the new therapy before it is made available to the public.
Once the treatment has passed these clinical trials, proper administration must be followed for therapeutic use.
This includes following a specific method for administration of the monoclonal antibody (i.e., parenterally, intravenously, subcutaneously, or intramuscular), and using the proper dose at specific time intervals to ensure maintenance of the monoclonal antibody concentration at appropriate levels.
Patients receiving monoclonal antibody treatment must also be observed for roughly an hour post-infusion to monitor for any adverse events.
This will allow for timely intervention should a reaction occur, giving the healthcare team sufficient time to prevent it from causing significant damage to the patient.
Molecular engineering technologies have also been developed to “fine-tune” the structure of monoclonal antibodies to improve their therapeutic actions while decreasing the risk of adverse effects, therefore increasing the risk-benefit ratio.
An example includes “humanizing” the monoclonal antibodies before administering them to the patient. Monoclonal antibodies are created by immunizing a specific animal with a target antigen, such as a rabbit.
B cell candidates that produce antibodies for this antigen are then harvested from the spleen cells of the rabbit, and an antibody with the desired specificity is chosen.
Large quantities of these monoclonal antibodies can then be produced for treatment therapies. This rabbit antibody production for use against human diseases runs the risk of causing an immune response, such as those described above, as these monoclonal antibodies are considered foreign by the human immune system.
To avoid this, amino acid substitutions in the monoclonal antibodies can be engineered using recombinant DNA protocols, making them similar to human sequences and decreasing the chance of an adverse event.
These protein-modifying technologies combined with vigorous clinical testing and safety measures during infusion in the patients help minimize the potential damage, showing that the potential benefits of these therapies far outweigh the risks.
Boster Bio is a company that specializes in developing custom rabbit monoclonal antibodies with high affinity and specificity tailored to the desired functionality.
They have shown to be highly successful in developing antibodies for difficult antigens with their patented microfluidics plasma cell discovery (PCD) platform, which allows for reduced screen time by beginning with the process of isolating antigen-specific plasma cells to ensure a larger selection of high-affinity antibodies.
For more information, please check out their Custom Rabbit Monoclonal Antibody Discovery Service.

